In the year 2020, headlines across the globe were captivated by a singular, bold act of scientific defiance. At the age of 49, Beata Halassy, a virologist at the University of Helsinki, refused to surrender to her third recurrence of breast cancer. Shunning the harsh regimens of chemotherapy, she turned instead to a treatment she believed in with all her scientific conviction: oncolytic virotherapy. Administering a self-designed therapy using lab-grown viruses, Halassy became both the creator and the subject of a revolutionary experiment. Four years hence, her cancer remains in remission, and her daring endeavor has reshaped the ethical landscape of medical science.
This dramatic tale is more than a scientific anecdote, it is the harbinger of a therapeutic frontier that marries genetic engineering with precision immunotherapy, leveraging viruses not as enemies, but as allies against cancer.
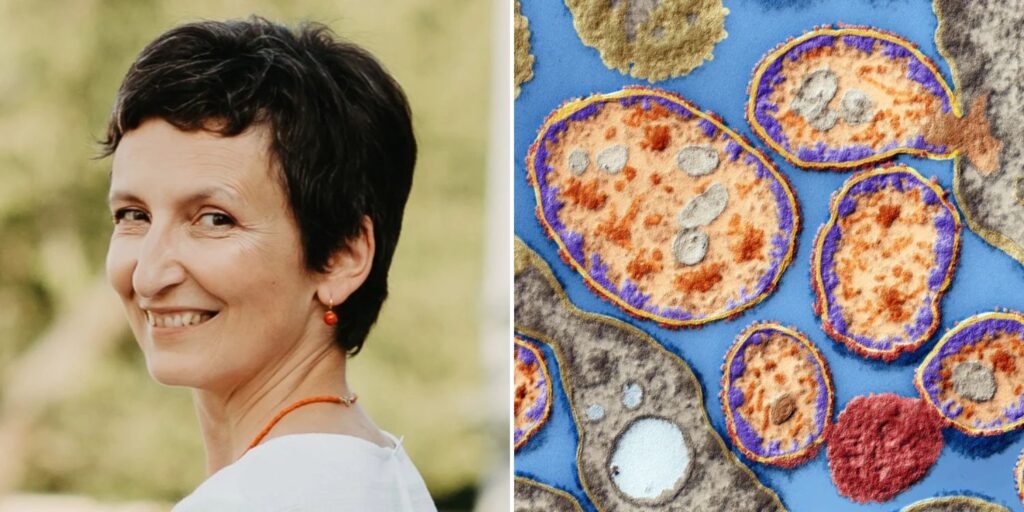
Image: Beata Halassy, from Croatia treats her own breast cancer with viruses she grew in lab. (Image source: mustsharenews.com)
Introduction to Oncolytic Virotherapy: Biological Assassins in Medicine
Oncolytic viruses (OVs) are genetically engineered or naturally occurring viruses that selectively infect and lyse tumor cells. Unlike traditional therapies that indiscriminately target both healthy and malignant cells, OVs replicate specifically within tumor cells, causing their rupture and subsequently exposing the internal tumor antigens to the immune system. This not only eliminates the infected cells but also catalyzes a systemic antitumor immune
response.
At the forefront of this therapy is Talimogene laherparepvec (T-VEC), derived from herpes simplex virus (HSV-1), the first FDA-approved OV therapy for metastatic melanoma. But this is merely the beginning. Ongoing clinical trials across the globe are refining OVs for an array of cancers—each designed as a precision-guided missile for oncologic warfare.
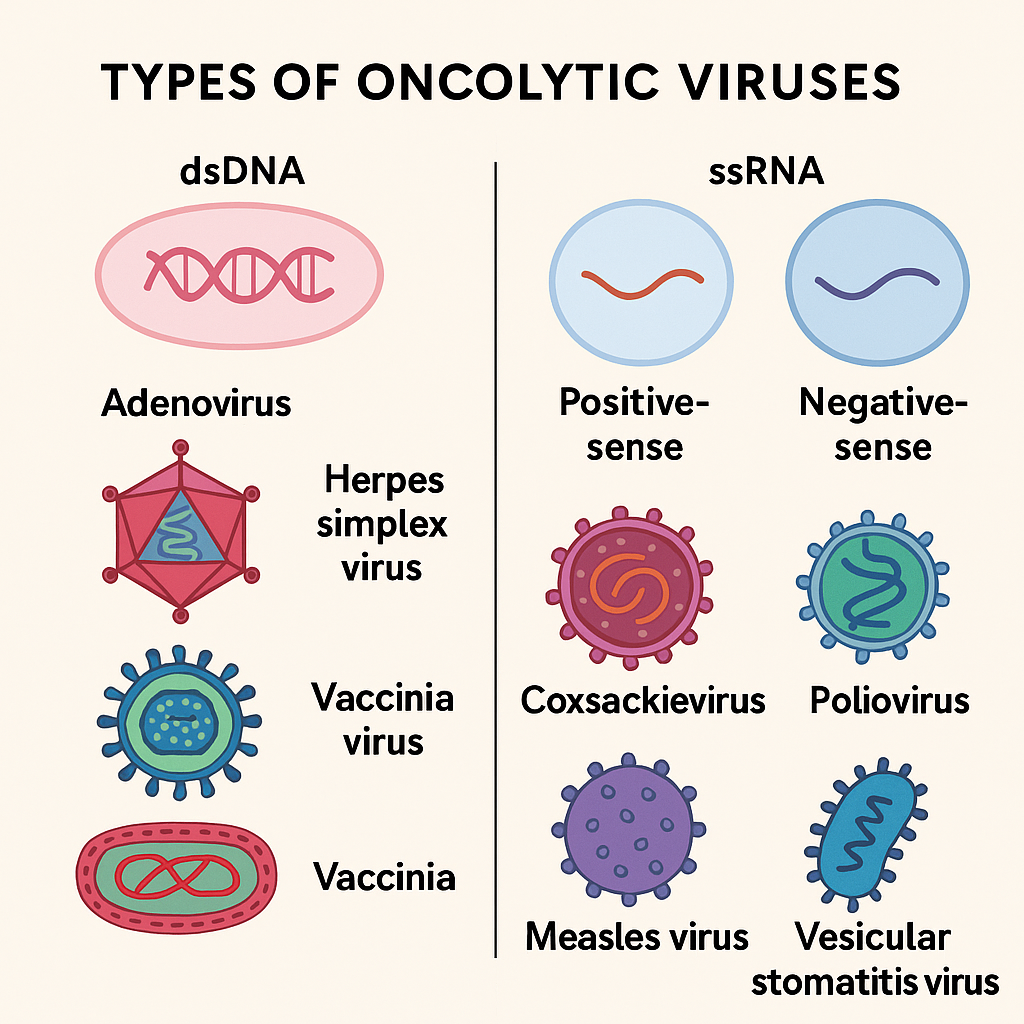
Types of Oncolytic Viruses: A Diverse Viral Arsenal
The arsenal of oncolytic viruses is as diverse as it is lethal to cancer. Based on their genetic material, OVs are classified into DNA and RNA viruses:
● Double-stranded DNA (dsDNA): Adenovirus, Herpes simplex virus (HSV), Vaccinia virus
● Single-stranded RNA (ssRNA): Positive-sense (Coxsackievirus, Poliovirus), Negative-sense (Measles virus, Vesicular stomatitis virus)
Prominent examples include:
● T-VEC (HSV-based): Engineered to express GM-CSF for immune activation.
● Reovirus: Targets Ras-activated pathways, showing promise in colorectal and
pancreatic cancers.
● JX-594 (Vaccinia virus): Armed with granulocyte-macrophage colony-stimulating
factor (GM-CSF).
● H101 (Adenovirus): Designed for p53-deficient tumors.
● Delytact (HSV-based): Approved in Japan for glioblastoma.
Each virus is tailored for specific malignancies, exploiting weaknesses in cancer biology to mount targeted strikes.
Mechanisms of Action: How OVs Wage War
Oncolytic viruses obliterate tumors through a series of intricate, multi-pronged strategies:
- Direct Tumor Lysis: By hijacking the defective antiviral machinery of cancer cells—such as impaired interferon (IFN) signaling—OVs replicate uncontrollably, causing rupture.
Lysosomal destabilization, caspase-3/-8/-9 activation, and TRAIL receptor (TRAILR1/TRAILR2) engagement culminate in apoptosis and necrosis. - Tumor Microenvironment (TME) Remodeling: OVs transform the immunosuppressive TME into a battleground. Damage-associated molecular patterns (DAMPs) like HMGB1, ATP, and calreticulin are released, recruiting dendritic cells (DCs) and cytotoxic T lymphocytes (CTLs). M2 macrophages are repolarized to pro-inflammatory M1 types, enhancing cytokine storms with IL-12 and IFN-γ.
- Antiangiogenesis: Tumor vasculature is decimated as OVs destroy endothelial cells and suppress angiogenic signals like VEGF and IL-8. Genetic inclusion of endostatin or angiostatin halts vascular growth.
- Metabolic Reprogramming: Tumor energetics, such as the Warburg effect and glutaminolysis, are sabotaged. Elevated reactive oxygen species (ROS) foster oxidative damage, collapsing the tumor’s metabolic scaffolding.
- Immune Activation: OVs act as vaccines, releasing pathogen-associated molecular patterns (PAMPs) that trigger Toll-like receptors (TLRs) on immune cells. Engineered expression of GM-CSF or IL-2 supercharges immune recruitment, reducing regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).

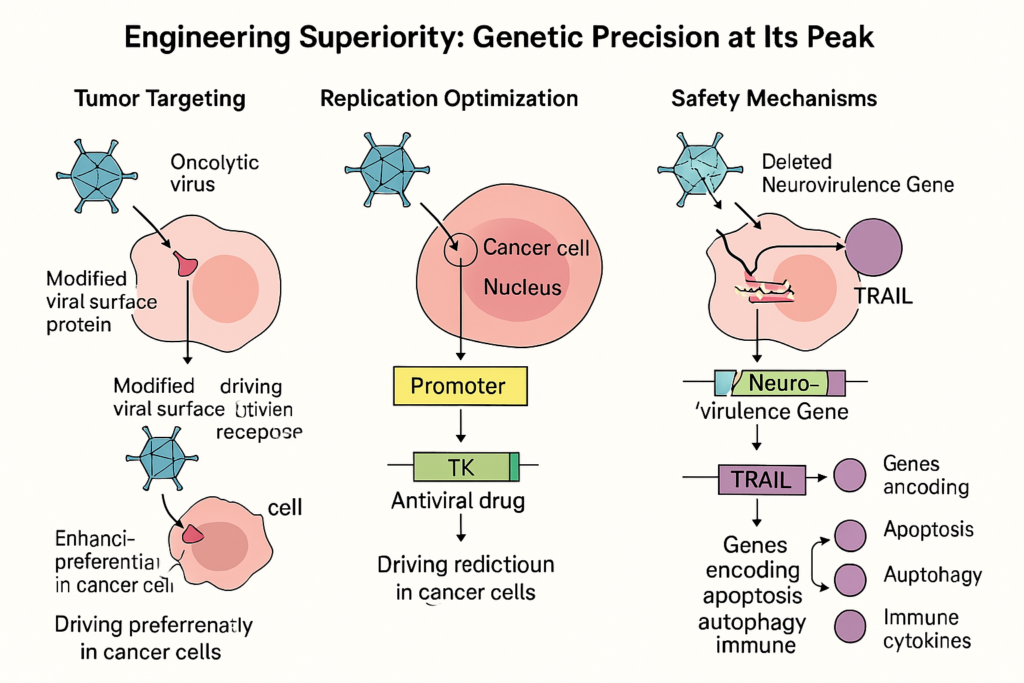
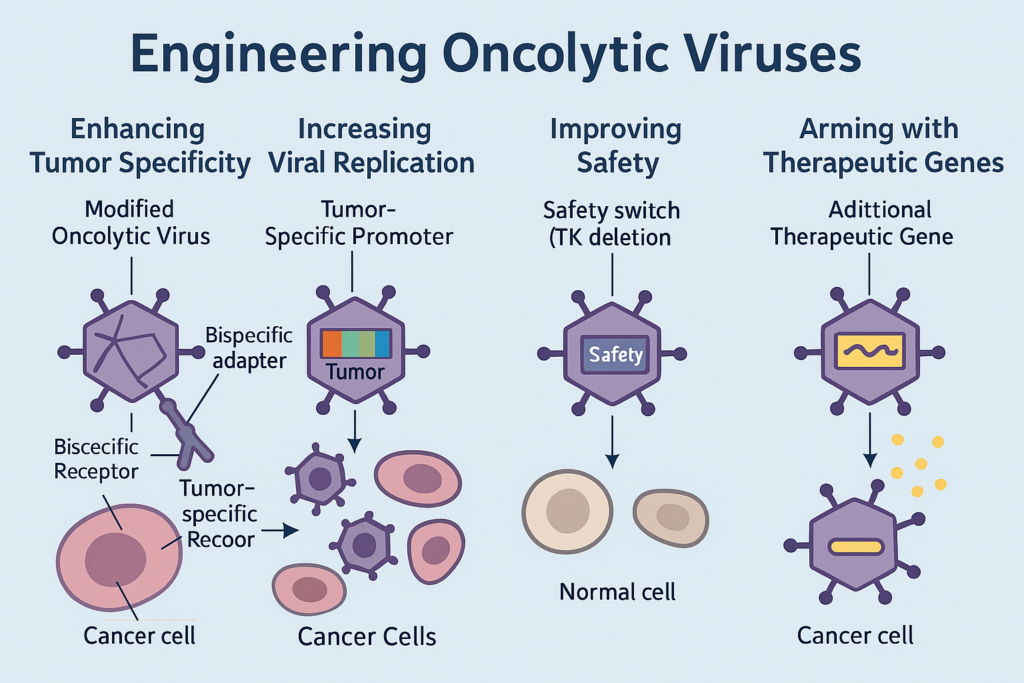
Engineering Superiority: Genetic Precision at Its Peak
The beauty of oncolytic virotherapy lies in its modularity. Genetic engineering allows enhancement of specificity, efficacy, and safety:
● Tumor Targeting: Modified viral surface proteins preferentially bind overexpressed tumor receptors like CD155 (poliovirus) or nectin-1 (HSV). Bispecific adapters or capsid swapping further finetune this targeting.
● Replication Optimization: Promoters like survivin or GP73 drive viral replication only in cancer cells. Deletion of the E1B-55kDa gene in H101 ensures activity only in p53-deficient tumors.
● Safety Mechanisms: Neurovirulence genes are deleted (as in HSV-1), and safety switches like thymidine kinase (TK) enable antiviral drugs to abort therapy if needed.
● Therapeutic Payloads: Genes encoding TRAIL, Beclin-1, or immune cytokines are inserted to enhance apoptosis, autophagy, and immune response.
An extraordinary example is a virus engineered to deliver a TGF-β inhibitor, as demonstrated by Dr. Delgoffe’s group. In mice models of resistant head and neck cancer, this virus significantly shrank tumors, showing synergy with checkpoint blockade and sparing systemic toxicity—marking a major leap in immunotherapy.
Clinical Impact and Challenges: A Double-Edged Sword
Despite promising results, several hurdles challenge OV therapy:
● Immune Clearance: Systemic delivery faces neutralization by pre-existing antibodies
or macrophages.
● Tumor Penetration: Dense extracellular matrices prevent adequate infiltration.
● Patient Variability: Tumor heterogeneity and immune profiles vary greatly.
However, combination strategies—pairing OVs with immune checkpoint inhibitors, chemotherapy, or radiotherapy—are overcoming these barriers. Predictive biomarkers and personalized virotherapies are on the horizon.
A Glimpse into the Future: Hope in Viral Bottles
The future of oncology could well be viral, not as a scourge, but as salvation. Imagine tailored viral vectors programmed to detonate inside cancerous tissues while broadcasting distress signals to the immune army. Picture self-amplifying therapeutics that evolve with their target, adjusting strategy mid-course.
With growing knowledge of genomic instability, immune checkpoints, and metabolic pathways, oncolytic viruses are being redesigned to out-think cancer. Clinical trials for glioblastoma, pancreatic cancer, ovarian carcinoma, and even resistant colorectal tumors are rapidly progressing. From Beata Halassy’s courageous act to the carefully regulated, biomarker-driven clinical platforms of today, the journey is nothing short of a scientific epic.
Conclusion: A Paradigm Rewritten
Oncolytic viruses are no longer a fantastical dream, they are tangible, programmable weapons in the war against cancer. Fusing molecular engineering with immunology, they exploit cancer’s vulnerabilities while rallying the body’s defenses. What was once a passive battlefield now pulses with active resistance.
As this modality matures, supported by global research networks, biotech innovation, and courageous pioneers, the war against cancer may finally tilt in humanity’s favor. And perhaps, years from now, patients will not dread chemotherapy but will instead embrace viruses—once feared, now revered—as saviors coded in life’s smallest scripts.
References:
- Corbyn, Z. (2024, November 9). This scientist treated her own cancer with viruses she grew in the lab. Nature. https://www.nature.com/articles/d41586-024-03647-0
- Volovat, S. R., Scripcariu, D. V., Vasilache, I. A., Stolniceanu, C. R., Volovat, C., Augustin, I. G., Volovat, C. C., Ostafe, M.-R., Andreea-Voichita, S.-G., Bejusca-Vieriu, T., Lungulescu, C. V., Sur, D., & Boboc, D. (2024). Oncolytic virotherapy: A new paradigm in cancer immunotherapy. International Journal of Molecular Sciences, 25(2), 1180. https://doi.org/10.3390/ijms25021180
- Mondal, M., Guo, J., He, P., & Zhou, D. (2020). Recent advances of oncolytic virus in cancer therapy. Human Vaccines & Immunotherapeutics, 16(10), 2389–2402. https://doi.org/10.1080/21645515.2020.1723363
- Lin, D., Shen, Y., & Liang, T. (2023). Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduction and Targeted Therapy, 8, Article 156. https://doi.org/10.1038/s41392-023-01407-6
- Bai, Y., Hui, P., Du, X., & Su, X. (2019). Updates to the antitumor mechanism of oncolytic virus. Thoracic Cancer, 10(4), 1031–1035. https://doi.org/10.1111/1759-7714.13043
- Russell, S. J., Peng, K. W., & Bell, J. C. (2012). Oncolytic virotherapy. Nature Biotechnology, 30(7), 658–670. https://doi.org/10.1038/nbt.2287
- Fukuhara, H., Ino, Y., & Todo, T. (2016). Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Science, 107(10), 1373–1379. https://doi.org/10.1111/cas.13027

It’s something new and exciting , Good research and amazing work