

(AI Generated image)
In 1976, the bustling corridors of a metal-processing plant in Belgium witnessed an extraordinary discovery. Among the industrial waste and metallic debris, a group of scientists identified a remarkable bacterium, later named Cupriavidus metallidurans. Unlike most microbes, which would perish in such toxic environments, this resilient bacterium thrived. It not only endured the heavy metal-laden surroundings but also detoxified harmful compounds, making it a scientific marvel. Decades later, in 2009, researchers uncovered another astonishing capability: C. metallidurans could convert toxic metals into 24-carat gold within just a week, a feat that captured global attention.
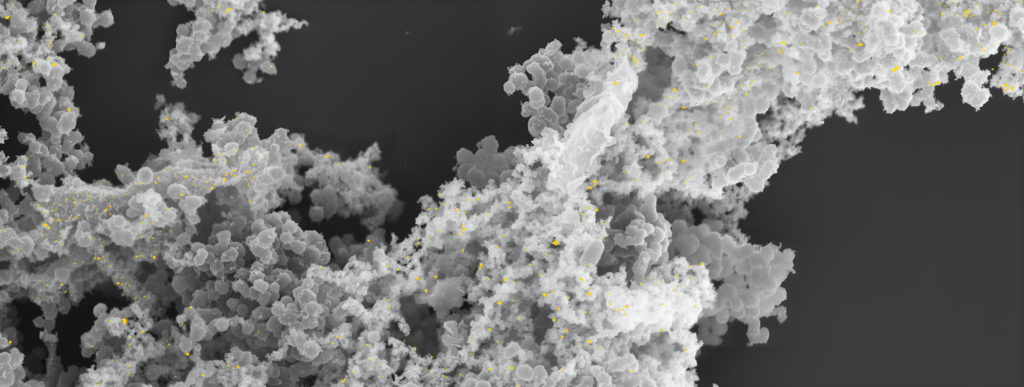
Fig: Production of gold by the bacteria in an engineered atmosphere contained within a customised alchemical bioreactor. (Credit: Adam Brown)
This small bacterium, with its robust survival mechanisms, quickly became a focal point in studies addressing metal detoxification and environmental cleanup. Its ability to withstand toxic heavy metals, including cadmium, opened new doors for addressing pollution-related challenges. In agricultural settings like rice fields, cadmium contamination has long posed significant threats. Industrial activities have led to the accumulation of this heavy metal in soil, making it a persistent issue. Cadmium exposure in humans causes severe health problems, such as kidney failure and cancer. In response to these dangers, organizations like the FAO and WHO have established strict limits on cadmium content in rice. Yet, the problem persists, fueling the urgent need for innovative, sustainable solutions.
Researchers around the world have turned to C. metallidurans as a potential ally in the fight against cadmium contamination. A team from the State Key Laboratory of Biocatalysis and Enzyme Engineering in Wuhan, China, took this a step further. They isolated a unique strain of the bacterium, named CML2, from rice seedlings exposed to cadmium in contaminated fields. Their goal was clear: to explore the bacterium’s potential for cadmium removal and its impact on soil health and food safety. Using modern molecular techniques like PCR amplification and bioinformatics tools, they identified C. metallidurans CML2 and conducted a series of experiments to understand its capabilities.
The results were nothing short of remarkable. The CML2 strain demonstrated exceptional resistance to cadmium, tolerating concentrations as high as 2400 mg/L. To put this into perspective, this level is far beyond what is typically found in natural environments. Such resilience positioned CML2 as a powerful candidate for bioremediation efforts in polluted agricultural lands.
The team didn’t stop at identifying the bacterium’s resistance. They worked meticulously to optimize its growth conditions. Through experiments involving temperature, pH, and salt concentration, they determined that C. metallidurans CML2 thrived best at 30°C, with a salt concentration of 1.0% and a pH range of 5.8 to 6.2. These conditions not only supported the bacterium’s growth but also enhanced its ability to detoxify cadmium. When exposed to cadmium chloride (CdCl2) over five days, CML2 removed about 319 mg/L of cadmium from
the environment, even under extreme stress.
To understand how C. metallidurans achieved this feat, the researchers used advanced imaging and molecular analysis tools. Scanning Electron Microscopy (SEM) revealed that the bacterium formed biofilms and nanoparticles as defense mechanisms against heavy metal stress. These biofilms acted as protective shields, while the nanoparticles facilitated the detoxification process. Further analysis with Energy Dispersive X-ray Spectroscopy (EDS) confirmed that cadmium adhered to the bacterial surface. Molecular tools like Fourier Transform Infrared Spectroscopy (FTIR) identified functional groups involved in cadmium binding, while X-ray Photoelectron Spectroscopy (XPS) provided insights into the chemical states on the bacterium’s surface.
The team then delved into the genetic mechanisms driving this resilience. By extracting RNA from CML2 and sequencing it, they identified over 700 differentially expressed genes. These genes, activated in response to cadmium exposure, enhanced the bacterium’s energy production and metal detoxification processes. A key discovery was the activation of the czcCBA gene cluster, which played a pivotal role in pumping cadmium out of bacterial cells.
This genetic response was crucial to the bacterium’s ability to withstand high cadmium concentrations and transform it into less harmful forms.
The real-world applications of these findings were tested through pot experiments. The researchers grew rice plants under three different conditions: in soil with cadmium alone, in soil with C. metallidurans alone, and in soil with both cadmium and the bacterium. The results were striking. While cadmium alone severely stunted the growth of rice plants, C. metallidurans mitigated these harmful effects. Rice plants grown in the presence of the bacterium showed improved root and shoot growth, as well as reduced cadmium uptake. This demonstrated that C. metallidurans could lower the bioavailable cadmium in the soil, offering a dual benefit of detoxification and crop protection.
In conclusion, Cupriavidus metallidurans CML2 emerges as a promising solution for addressing cadmium contamination in rice fields. Its ability to thrive under extreme conditions, detoxify cadmium, and promote plant growth underscores its potential as a bioremediation agent. As industrial pollution continues to jeopardize food production, the use of resilient bacteria like C. metallidurans offers hope for sustainable agricultural practices.
However, further research is needed to explore large-scale applications and unravel the detailed mechanisms behind its detoxification processes.
By integrating biological solutions into farming, we can pave the way for safer food systems and a healthier planet. The resilience and adaptability of C. metallidurans remind us of nature’s capacity to combat even the most daunting challenges, making it a true ally in the fight for environmental sustainability.
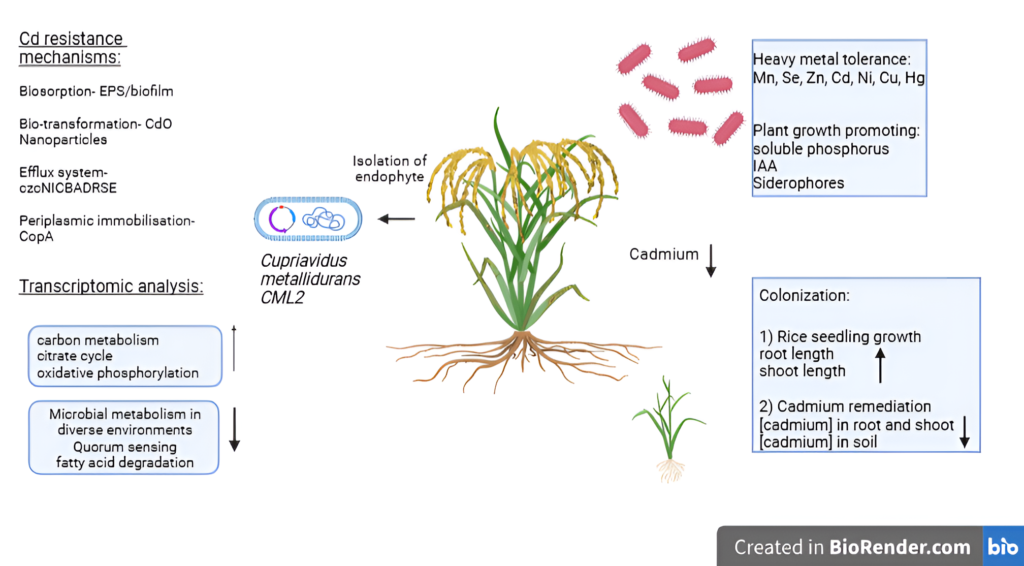
A cartoon representing “All about Cupriavidus metallidurans in bioremediation” (made with https://www.biorender.com/)
References:
- Nies DH. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999 Jun;51(6):730-50. doi: 10.1007/s002530051457. PMID: 10422221.
- Reith F, Etschmann B, Grosse C, Moors H, Benotmane MA, Monsieurs P, Grass G, Doonan C, Vogt S, Lai B, Martinez-Criado G, George GN, Nies DH, Mergeay M, Pring A, Southam G, Brugger J. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17757-62. doi:10.1073/pnas.0904583106. Epub 2009 Oct 7. PMID: 19815503; PMCID: PMC2764933.
- Van Houdt, R., et al. (2021). Adaptation of Cupriavidus metallidurans CH34 to toxic zinc concentrations involves an uncharacterized ABC-type transporter. Microorganisms, 9(2), 309. https://doi.org/10.3390/microorganisms9020309
- Zammit, C.M., Reith, F.(2013). Gold iomineralization in acterium Cupriavidus Metallidurans. In: Kretsinger, R.H., Uversky, V.N., Permyakov, E.A. (eds) Encyclopedia of Metalloproteins. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-1533-6_581
- Zhang, Y., et al. (2024). Endophytic bacteria for Cd remediation in rice: Unraveling the Cd tolerance mechanisms of Cupriavidus metallidurans CML2. Journal of Hazardous Materials, 133846. https://doi.org/10.1016/j.jhazmat.2024.133846

Very good finding .
It will definitely help in agricultural fields to improve crop production.
😊
Interesting!!! Can this bacteria be used in treatment of water pollution from heavy metal?
Yes, Cupriavidus metallidurans holds great potential for treating water pollution caused by heavy metals. Its natural ability to detoxify metals like cadmium, lead, and mercury makes it an excellent candidate for bioremediation in aquatic environments.
By introducing this bacterium into contaminated water systems, it can absorb and transform toxic heavy metals into less harmful forms or bind them to its cell surface. Research has shown that it forms biofilms and nanoparticles during this process, which enhance its metal detoxification efficiency. However, large-scale applications would require careful study to ensure the bacterium’s survival in diverse aquatic conditions and its long-term impact on the ecosystem.