
Vaccines are mainly used in preventing diseases because they are biological products designed to stimulate the immune system to generate Antigen-specific immunity against a pathogen.
When a vaccine is administered, the immune system recognizes certain components of the pathogen known as antigens, triggering a targeted immune response. Thus, the vaccine ‘trains’ to respond effectively to the pathogen upon exposure; this concept is referred to as immunological memory. Malaria is one of the most widespread diseases, as it affects 228 million people worldwide and is responsible for 405.000 deaths each year. In addition to this enormous burden of morbidity and mortality, malaria is also responsible for significant economic losses in endemic areas.
It is found in Central America and South-East Asia and most malaria is seen in sub-Saharan Africa. Of these, sub-Saharan Africa is the most affected region, accounting for approximately 85% of the world’s cases of the disease.Children under five years old and pregnant women are at serious risk of this disease. It also occurs early in people with low immunity.
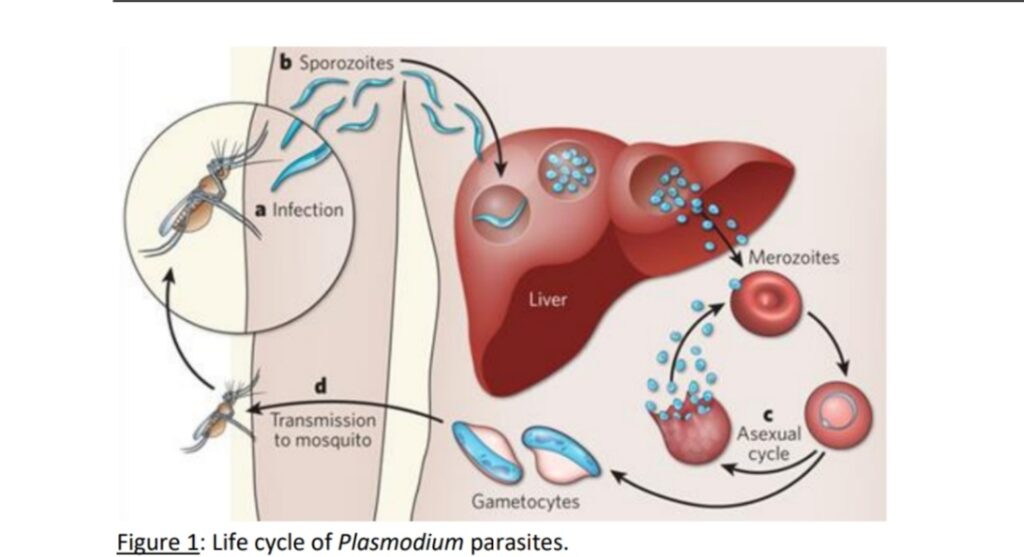
Malaria in humans is caused by five species of Plasmodium protozoa: Plasmodium falciparum (PF), Plasmodium ovale, Plasmodium vivax, Plasmodium malaria and Plasmodium knowlesi . Plasmodium parasites are transmitted to humans (definitive hosts) by female Anopheles mosquitoes. Also, we say that female Anopheles mosquitoes act as vectors to transmit the parasite to humans. Parasites are injected into the skin as sporozoites which then migrate to the liver. These parasites eventually enter the bloodstream as merozoites, invade red blood cells, and reproduce within them, leading to the characteristic cyclical fever episodes associated with malaria. The disease’s deadly impact arises primarily from organ failure caused by the sequestration of infected red blood cells in the body’s small blood vessels. Sporozoites infect hepatocytes, where they develop and multiply until they enter the bloodstream as merozoites, then invade erythrocytes for replication.
There are many treatment options for malaria, from chloroquine to the recently discovered artemisinin-derivatives, yet there is growing concern about their efficacy as resistance to chloroquine is already widespread and novel artemisinin-derivatives are on the rise. Currently, malaria is becoming increasingly difficult to treat, increasing the urgency of developing preventive tools. Examples of disease prevention strategies include bite avoidance and vector control measures, chemoprophylaxis, and vaccination.
• Available vaccines and their characteristics:
Some malaria vaccines have been certified by the World Health Organization (WHO).
Currently available vaccines use subunit vaccines. These vaccines give us some protection, but the effect is limited and effective for a short period of time. This has led to new policies and research Alternative vaccination strategies are being developed that attempt to increase protection by reducing the genetic makeup of the Plasmodium parasite.
For example, a genetically modified Plasmodium vaccine called GA1 stops the development of the parasite in the small intrahepatic stage. This leaves protection limited. In contrast, a vaccine called GA2 has shown promising results in research, but no clear conclusions have yet been drawn.
Previous tests and results: Vaccine efficacy has been modest in previous trials.
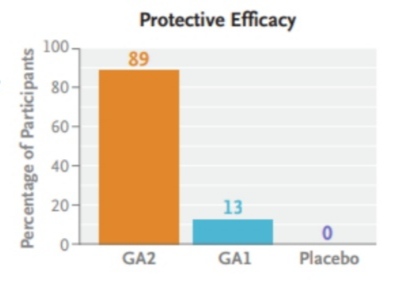
GA2 vaccine may be more effective than GA1, but this is still uncertain.
• Recently, a new GAP, GA2 parasite (Pf∆mei2), which lacks one gene and is developmentally arrested. Late liver-stage, was produced. The advantage of the GA2 parasite is that, by arresting development At a later stage than RAS and previous GAPs, it may allow prolonged antigenic exposure immune system and thus have more potent immunogenic properties.GA2 phenotype closely resembles a “sporozoites under chemoprophylaxis strategy”, whereby live sporozoites Chloroquine or mefloquine prophylaxis administered under cover, which has previously been shown to induce immunity highly efficiently in healthy malaria-naive individuals. The GA2 parasite would have good protective efficacy and would be a suitable candidate for a vaccine. The scope of this trial is to evaluate and test GA2 parasite safety and tolerability in humans. Its primary protective efficacy against controlled human malaria infection (CHMI). Moreover, the immunological responses elicited by the GA2 parasite will be compared to those elicited by one of its counterparts. How was the test conducted?
Stage A: Open-label dose-escalation
The test was conducted on adults who had not previously had malaria. The test participants were divided into three groups:
50 bites of GA2-infected mosquitoes
50 bites of GA1-infected mosquitoes
50 uninfected mosquito bites (placebo)
Stage B: Vaccination process
The participants were vaccinated through mosquito bites in three sessions at an interval of 28 days.
Three weeks after the last vaccination, participants were given controlled human malaria infection (CHMI) via 5 bites of mosquitoes infected with unattenuated P. falciparum strain 3D7 to test immunity.
Primary Objective:
Adverse events:
The number and severity of adverse events occurring among participants were recorded.
Successful transition:
Successful transmission by GA2 mosquito bites was measured by the presence of blood-stage parasites.
This trial examined the safety, tolerability, and effect of the GA2 vaccine on primary infection.
• Advances in Malaria Vaccines: An Approach
Malaria is one of the most serious diseases worldwide. Currently, the most advanced vaccine is called RTS, S/AS01. The vaccine is based on a major protein (CSP) from the malaria parasite. It is currently being used as part of a pilot project in three African countries (Malawi, Ghana, and Kenya). However, this vaccine provides only partial protection, and its effectiveness decreases over time. Therefore, a more effective vaccine is still needed.
• Need for new technology
The Malaria Vaccine Technology Roadmap has set a target of 75% efficacy. Some new approaches are being developed for this, such as:
• Attenuated sporozoite vaccine (ASP):
It involves taking sporozoites directly from the salivary glands of mosquitoes.
This prepares the body’s immune system to fight against a wide range of antigens from the parasite.
RAS (Radiation-Attenuated Sporozoites) is one of the ASP vaccines, under which the parasites are inactivated. They reach the liver cells but cannot grow their Benefits of ASP vaccines and comparison with other vaccines. ASP vaccines developed from Plasmodium sporozoites (attenuated sporozoites) activate the immune system longer and more effectively. This strengthens the immune response against various antigens in the liver. This is because it contains a wide range of antigens that affect different stages of the Plasmodium life cycle.
• RAS Vaccination (Radiation-Attenuated Sporozoites)
How does it work?
Sporozoites weakened by radiation after entering the body migrate to the liver and enter cells, but are unable to replicate.
Advantages:
Long-lasting protection noted in mice,
monkeys, and humans.
Limitations: High doses are required for effective protection (at least 1000 sporozoites).
• CPS Vaccination (Chemoprophylaxis with Sporozoites)
How does it work?
Viable, replicating sporozoites are provided but are stopped by the drug before reaching the erythrocytic stage.
Advantages:
Low doses (35-46 sporozoites) are also effective.
Capable of long-term protection for up to 28 months.
Limitations: Not safe for large-scale use, due to risk of contact with live parasites.
• CPS VS RAS
CPS vaccines are more effective than RAS because the natural development of parasites in CPS allows the immune system to be exposed to different antigens for longer periods.
• New Solution: GAP Vaccination (Genetically Attenuated Parasites)
How does it work?
GAP vaccines aim to produce parasites that stop developing in the liver and do not reach the erythrocytic stage.
Advantages:
A safe and effective, but less risky alternative to RAS and CPS.
Conclusion:
CPS vaccine is highly effective, but not practical for safety reasons. GAP and ASP vaccines may be a safe and long-term option for the future. GA1 parasites: initial efforts
How does it work?
GA1 inhibits the development of the parasite in the early stages of the liver, thereby preventing infection in the blood.
Results:
It is safe in preclinical and clinical studies. A dose of 9×10⁵ was administered intravenously to healthy subjects.
Limitations:
Limited protective effectiveness. This led to the need to find more effective solutions.
GA2 Parasite: An Improved Version
How does it work?
GA2 arrests development in the late stages of the parasite liver. This exposes the immune system to antigens for a longer period of time, leading to a stronger and longer-lasting immune response.
GA2 is intended to be more effective than RAS while maintaining a similar effect to the CPS vaccine.
Design of the GA2 parasite
How is it produced?
GA2 NF54 is produced by removing the mei2 gene from the parasite genome.
This gene is important for late-stage liver parasite replication and the formation of merozoites.
About NF54
This parasite was isolated from a historical case of malaria (‘airport malaria’ in the Netherlands).
It is of West African origin.
NF54 and its clones (e.g., 3D7) have previously been used in several malaria vaccines.
Preclinical findings
In Vitro Experiment:
GA2 invaded human liver cells.
But, after 6-7 days its development stopped completely.
Testing in animal models:
In mice with human liver and blood cells, GA2 did not induce blood infection.
This proved to be safe.
GA1 Vs. GA2: Comparison
GA1 parasite:
It inhibits the development of the liver at an early stage but with limited protective efficacy.
GA2 parasite:
Extends to the late stages of the liver, keeping the immune system active longer.
Next steps: Human trials for GA2
These trials will compare the safety, tolerability, and protective efficacy of GA2 with GA1.
Mode of Administration:
GA1: Administered intravenously as a cryopreserved product.
GA2: The test will be done using the MB (Multiple Bytes) model initially, then processed for GMP production.
conclusion
GA2 could be the next big step in parasite vaccination. Due to its advanced design, it may offer a more effective and safer vaccination option than previous GAPs. Now it is important to test its effectiveness on humans.
References:
1. A guide to vaccinology: from basic principles to new developments Andrew J Pollard, Else M Bijker Nature Reviews Immunology 21
2. World Health Organization. World malaria report 2021
3. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386:31
4. New England Journal of Medicine
Volume 391 • Number 20 • November 21, 2024
Pages: 1913-1923
5.Roestenberg M, Walk J, van der Boor SC, et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med 2020;12(544):eaaz5629-eaaz5629.
6.Hoffman SL, Goh LML, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002;185:1155-1164.
7. https://www.nejm.org



