Cancer Immunosurveillance and Immune Evasion
Surveillance by the immune system is a process by which the host immune system (innate and adaptive) actively surveys the body from within. Immunosurveillance describes the process whereby the immune system recognises pre-cancerous cells and proposes that the immune system can identify and destroy cancer precursors in most cases. This process involves the detection and destruction of cancer cells by various immune cells such as Cytotoxic T lymphocytes (CTLs), Natural Killer cells (NK cells), Antigen presenting cells (APC). These immune cells recognise and reduce the tumor growth. In response, cancer cells have evolved a variety of defence mechanisms known as “immune evasion”, a characteristic Hallmark of cancer, to thwart or inhibit immune-mediated targeting and destruction. Immune evasion mechanisms such as antigen escape, modulating antigen presenting molecules, secretion of immune inhibitory cytokines and recruiting immunosuppressor cells in tumor microenvironment (TME). One of the most crucial strategies to decrease the immune response by cancer cells is the recruitment and functional execution of regulatory T-cells (Tregs).
Tregs and its functions
Tregs are specialised subset of CD4+ T cells that are characterized by the expression of FoxP3 (Forkhead Box P3) transcription factor and CD25 which are required for its progression, maintenance and immunosuppressive function (4). Under physiological conditions, Tregs have crucial functions such as inducing oral tolerance to dietary antigens, suppressing allergies and asthma, promoting maternal-fetal tolerance, and preventing autoimmune disorders by assuring self-tolerance. They also restrict T-cell activation, control immunological responses, and lessen pathogen-induced immunopathology. Additionally, Tregs offer feedback to regulate the strength of the immune response, prevent commensal microorganisms, and inhibit T-cells from mistakenly using low-affinity TCR ligands to target self-like molecules. Their critical function in immune control and homeostasis is highlighted by these intricate systems. However, Tregs play dual functions in the context of cancer. They play a major role in tumor growth even though they are essential for avoiding chronic inflammation, which can fuel oncogenesis. Tregs inhibit anti-tumor immune responses in the tumor microenvironment (TME), which allows tumor cells to multiply and avoid apoptosis. Tregs are positioned in this dual role as both collaborators in cancer’s immune evasion tactics and defences against inflammatory damage.
Mechanisms of Treg in Tumor Microenvironment
In order to promote immunosuppression and tumor progression, the initial Treg accumulation in the TME is very crucial. Tumor cells, stromal cells, etc produces chemokines such as CCL17, CCL22, CCL28, CXCL9, CXCL10 and CXCL11 in TME. Treg is a chemoattractant which expresses chemokine receptors is bound by respective chemokines and direct the migration of Tregs to the TME. Thymus-derived Tregs, recognising self-antigens via high-affinity TCRs, encounter abundant tumor-associated antigens from dying tumor cells, leading to clonal expansion and enhanced suppressive activity. Tregs are further stabilised and activated by immunosuppressive cytokines released by tumor cells or any immunosuppressor cells. Furthermore, Inducible T-cell co-stimulator (ICOS) on Tregs promotes the growth of plasmacytoid Dendritic cells (pDCs) by binding to its ligand on pDCs. Together, these processes guarantee Treg accumulation and maintain the immunosuppressive properties of TME, which promote immune evasion and tumor growth.
IL-2 is primarily produced by the Effector T-cells (Teff cells) in the TME for their own proliferation and survival. IL-2 helps in the maintenance of the Teff to combat against tumor cells. Tregs in the TME expressing CD25 (alpha chain of IL-2 receptor) has high affinity towards the IL-2. So, Tregs consume IL-2 in the TME for their own induction and inhibiting the proliferation and survival of Teff cells. The hijacking of the IL-2 is one the major immunosuppressive mechanisms is shown by Tregs.
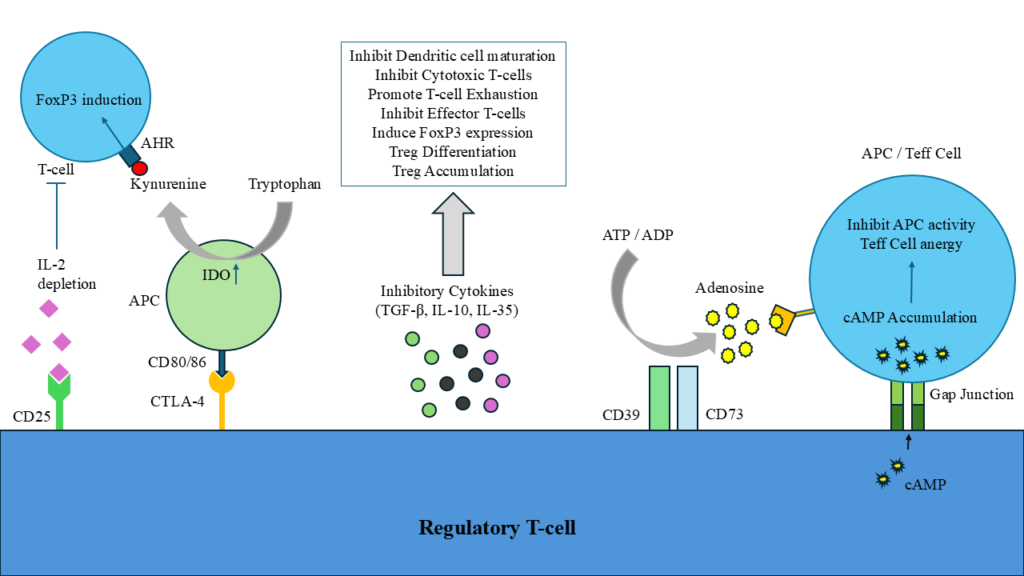
CTLA-4 is one of the important Immune checkpoints (ICs) expressed by Tregs. CTLA-4 basically binds to CD80/86 complex of antigen presenting cells (APCs). CTLA-4 acts as a competitor of CD28 expressed on T-cells. As CTLA-4 has higher affinity than CD28 towards CD80/86, it inhibits the binding of CD28 to CD80/86. The binding of CTLA-4 to CD80/86 results in the production of high Indoleamine 2,3-dioxygenase (IDO) inside the APCs. The IDO is a catabolic enzyme which metabolises the Tryptophan in APCs to form Kynurenine. The depletion of tryptophan results in altering the function of APCs and the kynurenine formed has been identified as the endogenous ligand for the aryl hydrocarbon receptor (AHR). Ligation of the AHR results in the promotion of Tregs by inducing FoxP3 expression and the suppression of normal T-cells. Another example is expression of PD-1 by Tregs bind to PD-L1 expressed on T-cells and inhibits the activation of T-cells or APCs by sending apoptotic signals. TIGIT, LAG-3, TIM-3 are also few important examples of ICs help in immunosuppressive nature of Tregs as measured by high secretion of IL-10, perforin and granzymes.
Within the TME, Tregs are known to produce inhibitory cytokines such as IL-10, IL-35 and TGF-β that are essential for immunosuppression and tumor growth. IL-10 promotes the tumor progression by preventing dendritic cell maturation, suppressing cytotoxic T-cell activity, and lowering the production of effector molecules including IFN-γ granzyme B. Similarly, Treg-secreted IL-35 increases inhibitory receptors on Teff cells, including PD-1, TIM-3 and LAG-3, hence promoting T-cell exhaustion. Additionally, it causes cell cycle arrest, which inhibits Teff function and proliferation. By promoting the exhaustion of tumor-infiltrating T-cells and boost the suppressive activity of Tregs, IL-10 and IL-35 work together to inhibit anti-tumor immune responses and produce an immunosuppressive environment that facilitates tumor immune evasion. Despite of these two crucial cytokines, Tregs produce high TGF-β, a key mediator, in the induction of FoxP3 expression. It is essential for Treg differentiation, maintenance and immune suppression. This cytokine can influence the composition and function of tumor-infiltrating lymphocytes (TILs), prompting Treg accumulation and enhancing Treg-mediated immunosuppression.
Tregs also suppress the immune responses in TME involving cyclic AMP (cAMP). Tregs express ectoenzymes called CD39 and CD73 which are essential to convert the extracellular ATP and ADP into adenosine formation. Adenosine molecules have high affinity towards the A2a receptors expressed by APCs and Teff cells. Adenosine activates high-affinity A2a receptors which results in the production of cAMP intracellularly via adenylyl cyclase 9 (AC9). The increased production of cAMP disrupts the metabolism in the Teff cells and causes anergy of Teff cell thus impairing immune responses. Furthermore, Tregs can directly transfer their intracellular cAMP into Teff cells and APCs through gap junctions mediated by connexin-43 and further amplification of cAMP into these immune cells. These cAMP inhibits the TCR-mediated signaling pathway by preventing the phosphorylation of ZAP70 (zeta-chain associated protein kinase 70). This results in reducing the proliferation, activation and survival of Teff cells and APCs in TME and leads to aggressive immunosuppressive nature of Tregs.
In conclusion, regulatory T cells are a double-edged sword in our immune system. Under normal conditions, they are essential for maintaining balance, preventing autoimmunity, and ensuring self-tolerance. However, in the tumor microenvironment, their role takes a darker turn. Tumors exploit Tregs to suppress the immune system, allowing cancer cells to evade detection and grow unchecked. Tregs create a shield that protects tumors from immune attacks. This hijacking of their natural function highlights the complexity of the immune system and its role in cancer progression. Moving forward, targeting Tregs in the tumor microenvironment offers a promising opportunity to break this protective barrier and restore the body’s ability to fight cancer. Balancing this approach carefully could unlock new possibilities for improving cancer treatment and patient outcomes.
References
1. Corthay, A. (2009). How do regulatory T cells work? Scandinavian Journal of Immunology, 70(4), 326–336. https://doi.org/10.1111/j.1365-3083.2009.02308.x
2. Ghorani, E., Swanton, C., & Quezada, S. A. (2023). Cancer cell-intrinsic mechanisms driving acquired immune tolerance. Immunity, 56(10), 2270–2295. https://doi.org/10.1016/j.immuni.2023.09.004
3. Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M., & Toda, M. (1995). Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology (Baltimore, Md. : 1950), 155(3), 1151–1164.
4. Saleh, R., & Elkord, E. (2020). FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Letters, 490, 174–185. https://doi.org/10.1016/j.canlet.2020.07.022
5. Togashi, Y., Shitara, K., & Nishikawa, H. (2019). Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nature Reviews Clinical Oncology, 16(6), 356–371. https://doi.org/10.1038/s41571-019-0175-7
6. Wang, Y., Li, J., Nakahata, S., & Iha, H. (2024). Complex Role of Regulatory T Cells (Tregs) in the Tumor Microenvironment: Their Molecular Mechanisms and Bidirectional Effects on Cancer Progression. International Journal of Molecular Sciences, 25(13), 7346. https://doi.org/10.3390/ijms25137346
7. Whiteside, T. L. (2015). The role of regulatory T cells in cancer immunology. ImmunoTargets and Therapy, 4, 159–171. https://doi.org/10.2147/ITT.S55415
8. Zhao, H., Liao, X., & Kang, Y. (2017). Tregs: Where We Are and What Comes Next? Frontiers in Immunology, 8. https://doi.org/10.3389/fimmu.2017.01578
9. Zitvogel, L., Tesniere, A., & Kroemer, G. (2006). Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature Reviews Immunology, 6(10), 715–727. https://doi.org/10.1038/nri1936



