
Introduction to Cancer and its Treatment
Cancer is one of the major health problems all over the globe with millions of new cases every year. Various treatment options to treat cancer are available such as Radiotherapy, surgery, chemotherapy. But these therapies have limitations and side effects which must be overcome by better strategies. Immunotherapy, a better therapy option, has been proven. Immunotherapy is a treatment that uses the immune system of the patient to counterattack the cancer. Among the immunotherapies, Chimeric Antigen Receptor (CAR) T-cell Therapy is the most efficient with less limitations observed in treating cancer. In this therapy, T-cells are extracted from the patient and then genetically modified to express the CAR specific to the target antigen of the cancer cell.
Components of CAR
CAR is a synthetic receptor consisting of four important regions.
1) Single Chain Variable Fragment (scFv) – The scFv is an extracellular antigen binding domain of the CAR derived from the Variable heavy (VH) and Variable light (VL) chains of the monoclonal antibodies connected via flexible linker. It is specific to the antigen present on the surface of the cancer CAR.
2) Hinge Region – Also known as the Spacer Region, it is extracellularly present that extends from antigen binding domain to the transmembrane domain. This reduces steric hindrance by providing flexibility and helps the antigen binding domain to freely bind to the specific antigenic epitope.
3) Transmembrane Domain – Transmembrane (TM) Domain embedded into the membrane of the CAR T-cell and anchors the CAR to the T-cell. This domain helps with stability and expression of CAR and dimerizes the endogenous molecules during intracellular signaling. TM is mainly derived from CD28, CD3ζ, CD4 or CD8α.
4) Intracellular Domains – Based on the generation of CAR, the number of Intracellular Domains differs. The first-generation CAR contains a single intracellular CD3ζ signaling domain next to TM, while the second-generation CAR contains one additional co-stimulatory domain (CD28 or 4-1BB) to the intracellular signaling domain. The third-generation CAR contains two co-stimulatory domains next to intracellular CD3ζ signaling domain while the fourth-generation CAR contains one intracellular CD3ζ signaling domain, two co-stimulatory domains and additional intracellular domain. This co-expresses certain small molecules that can induce cytokines or inhibitory molecules that can enhance anticancer effects and T-cell activation and proliferation.

Mechanism of CAR T-cell in Tumor Elimination
When CAR T-cell comes in the vicinity of the tumor, it first recognizes the antigen and binds with high specificity to antigen via scFv domain. The antigen can be a tumor-specific or tumor-associated present on the surface of the tumor cell. The binding of the CAR to antigen leads to the activation of the intracellular domain and initiates the cascade of signals as same as natural T-cell. This leads to the destruction of the tumor cells through various multiple processes. (1) CAR T-cells will release cytotoxic molecules such as perforins and granzymes leading to apoptosis of tumor cells. (2) CAR T-cells can also secrete the cytokines and enhance immune activation and anti-tumor effects. (3) CAR T-cells can stimulate and activate the recruitment of Natural T-cells and macrophages to attack tumors.

(Image Source: acgtfoundation.org)
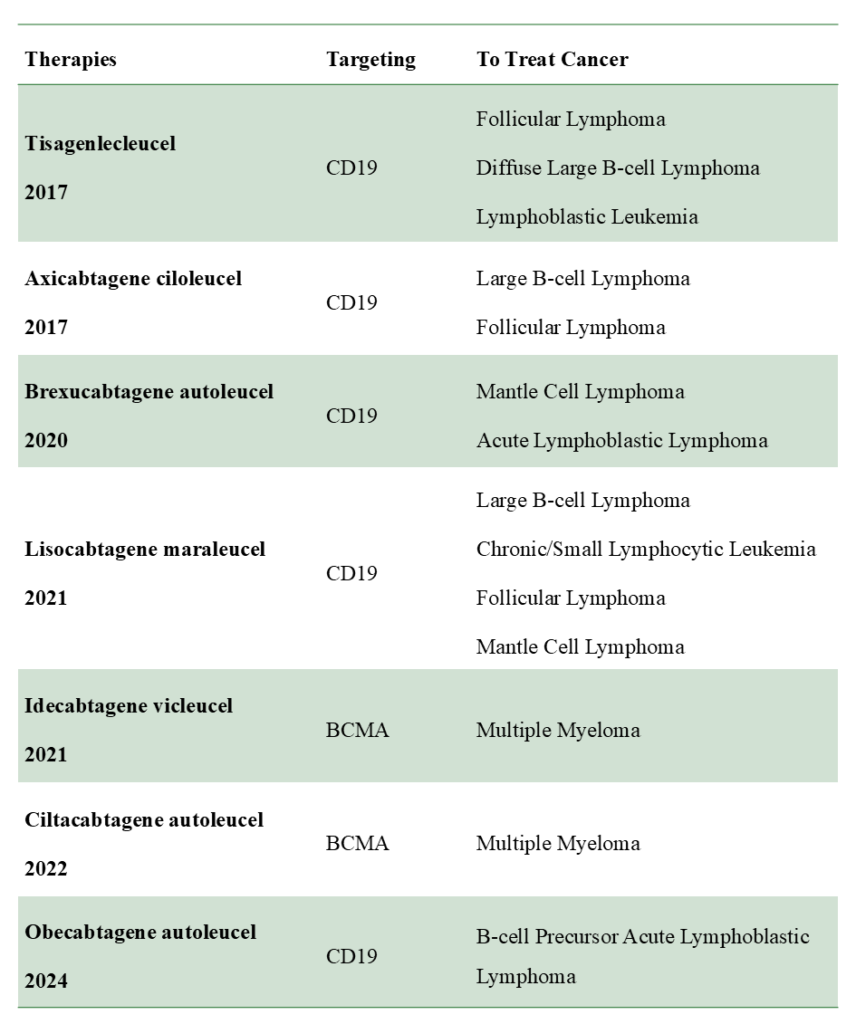
Approved CAR T-cell Therapies
Till now, the FDA has approved seven CAR T-cell therapies targeting only two antigens CD19 and BCMA, for treating specific types of Blood cancer and are listed below in the table.
Made in India CAR T-cell Therapy
The approval of NexCAR19 on October 13, 2023, marks a major milestone in India’s advancement in CAR-T cell therapy. Developed by ImmunoACT in collaboration with IIT Bombay, it provides a cost-effective treatment for leukemia and relapsed/refractory lymphoma. With a treatment cost of $30,000–$40,000, NexCAR19 is significantly more affordable compared to commercial CAR-T therapies in the United States, which range from $370,000 to $530,000, excluding hospital expenses and supportive care. Early clinical trials have shown encouraging results, with 19 out of 33 patients (58%) achieving complete tumor disappearance within one month, while four others (12%) experienced a 50% tumor reduction, leading to an overall response rate of 70%. As trial participants continue to be monitored for at least five years, NexCAR19 represents a transformative step in cancer immunotherapy, bringing new hope to patients in India and beyond. With ongoing long-term monitoring and further research, NexCAR19 has the potential to redefine cancer immunotherapy in India and beyond, offering hope to thousands of patients who previously had limited or unaffordable treatment options.
References:
1. Sterner, R.C., Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). https://doi.org/10.1038/s41408-021-00459-7
2. https://acgtfoundation.org/for-patients/approved-cell-and-gene-therapies/
3. Sun, D., Shi, X., Li, S., Wang, X., Yang, X., & Wan, M. (2024). CAR T cell therapy: A breakthrough in traditional cancer treatment strategies (Review). Molecular Medicine Reports, 29, 47. https://doi.org/10.3892/mmr.2024.13171
4. Radhakrishnan, V. S., Agrawal, N., Bagal, B., & Patel, I. (2021). Systematic Review of the Burden and Treatment Patterns of Adult and Adolescent Acute Lymphoblastic Leukemia in India: Comprehending the Challenges in an Emerging Economy. Clinical lymphoma, myeloma & leukemia, 21(1), e85–e98. https://doi.org/10.1016/j.clml.2020.08.023
5. Dwivedi, A., Karulkar, A., Ghosh, S., Srinivasan, S., Kumbhar, B. V., Jaiswal, A. K., Kizhakeyil, A., Asija, S., Rafiq, A., Kumar, S., Nisar, A., Patil, D. P., Poojary, M. V., Jain, H., Banavali, S. D., Highfill, S. L., Stroncek, D. F., Shah, N. N., Fry, T. J., Narula, G., … Purwar, R. (2021). Robust Antitumor Activity and Low Cytokine Production by Novel Humanized Anti-CD19 CAR T Cells. Molecular cancer therapeutics, 20(5), 846–858. https://doi.org/10.1158/1535-7163.MCT-20-0476
6. https://www.cancer.gov/news-events/cancer-currents-blog/2024/nexcar19-car-t-cell-therapy-india-nci-collaboration
7. https://www.immunoact.com/nexcar19



